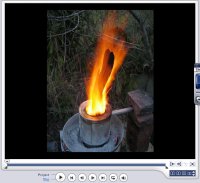
Hot stuff. My turbo furnace.
March 2007.
Please read my turbo-stove pages first. In a turbo-stove we want to heat food or water above the stove. In a metal furnace we want high temperatures in the combustion chamber. The stoves seem to reach around 1000 deg C, the furnace is my attempt to push that up to steel melting temperatures of >1400C. The exact melting point varies with carbon content.
 The
main difference between the stove and the furnace - is that in the
stove cold air is forced into the chamber - in the furnace the hot
air is used instead. Heat being exhausted out at the top is heat
wasted. To try to recover this heat I use a crude, counter flow,
heat exchanger.
The
main difference between the stove and the furnace - is that in the
stove cold air is forced into the chamber - in the furnace the hot
air is used instead. Heat being exhausted out at the top is heat
wasted. To try to recover this heat I use a crude, counter flow,
heat exchanger.
What happens is air is forced into the large
holes seen in this photo. Note well that the holes are only
through the outer layer of metal. The cold air travels down inside
the hollow double wall and is heated by the red hot inner surface.
The gasses inside the stove are rising and therefore “counter”
the direction of the incoming air. The hot air then enters the
combustion chamber through holes punched into the bottom of the
chamber.
Air is pumped into a manifold made from a milo tin. This is visible in the first photo. The air is fed in through a short piece of stainless steel pipe which is simply slit, spread and pop-riveted into the tin. The pump is an air-bed inflater.
The furnace makes it's own charcoal
fuel from wood. As seen in the picture I just used some twigs and
branches from my yard.
The first phase is “flaming
pyrolysis” - this is when the volatile components are burned
off.
In the second phase - (after the volatiles have gone) what
remains is a glowing charcoal bed.
Like the turbo-stoves - the furnace
cleanly burns the volatiles. Mainly CO2 is produced but since the
fuel is home grow wood the process is carbon neutral. Commercial
charcoal a is often made through much dirtier processes and may
also be made from fossil fuel.
BBQ charcoal is also
unattractive because it produces a lot of ash.
Ideally one
would do something useful with the flame during the first phase,
if nothing else it is entertaining.
The flame was hot enough to
heat metal to cherry red, hot enough to melt aluminium at least.
In any case the amount of fuel involved is minimal, I guess I used a kilo of wood here.
Also visible in the first photo is my insulation – I simply placed the flask in a bed of wood-ash. We can make our own insulation as well :)
 This
is the result of my first attempt.
This
is the result of my first attempt.
I wasn't too surprised the flask melted.
What did surprise me was that it burned!
The black stuff here isn't charcoal, it is heavy and attracted to magnets – it is iron oxide.
 I
was using a steel skewer to see if I'd reach steel's melting
point. This was a little bit short so I may not have been reaching
to hottest part of the fire. I melted some copper but didn't look
inside to see it happen.
I
was using a steel skewer to see if I'd reach steel's melting
point. This was a little bit short so I may not have been reaching
to hottest part of the fire. I melted some copper but didn't look
inside to see it happen.
I remember the metal igniting but didn't know was going on at the time. The flask move a bit and the sounds changed. The fire which was already white hot seemed to intensify and white sparks blew out through the top. It reminded me of steel making furnaces I'd seen on TV. There was also a strange smell.
I had a sort of “is she going to blow” feeling but when everything seemed stable I tested again with the skewer. It appeared to have melted at the tip and on the end was stuck what appeared to be molten metal. When stuff is that hot you just see the glow not the real colour. The skewer was slightly melted but on cooling the bulk of the material was black oxide. If it really was molten ferric oxide then the temperature reached 1565 deg. I'm not convinced that is the case because stainless steel is an alloy so other metals are present along with the big dose of copper I'd added. Copper oxide melts at only 1201 deg but I would have expected green flames if the copper burned.
Melting steel is just a novelty. I'll attempt it again in a month or so with some sort of refractory lining in place – possibly just a clay pot. I'm more interested in melting aluminium and that is easy.
More later....